electronic configuration of co|Iba pa : Clark Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa Ang una kong napansin ay ang bulbol ni tatay. Medyo malago ang bulbol nya at may linyang paakyat sa kanyang pusod. Pati ang itlog nya ay may kaunting bulbol at ang hita nya ay may kaunting buhok din. Ang malambot na titi ni tatay ay mas mahaba at mas mataba pa kesa sa matigas kong titi. Ang bayag nya ay nakabitin, halos kasing haba na .
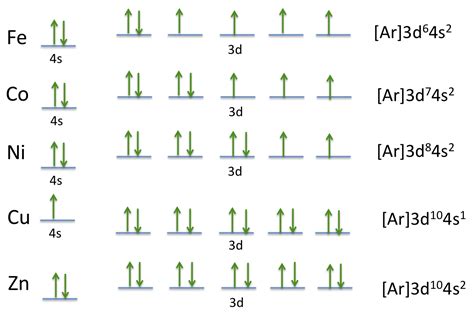
electronic configuration of co,The ground state electron configuration of cobalt is 1s2 2s2 2p6 3s2 3p6 3d7 4s2. This electron configuration shows that the last shell of cobalt has two electrons and the d-orbital has a total of seven electrons. Therefore, the valence electrons of cobaltare nine. There are two types of cobalt ions. The . Tingnan ang higit paThe total number of electrons in cobaltis twenty-seven. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in cobalt in specific rules in different orbits and orbitals is called the electron . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit pa
electronic configuration of co Iba pa Mar 23, 2023 To write the configuration for the Cobalt ions, first we need to write the electron configuration for just Cobalt (Co). We first need to find the number of electrons .

Full Electron Configuration For Cobalt. Full electron configuration can be defined as 27 electrons distribution in 4 shells of Co element. There are 2, 8, 15, 2 elements present in the 4 orbits of . The electronic configuration of cobalt is: 1s2 2s2 2p6 3s2 3p6 3d7 4s2. Cobalt has an atomic number of 27, which means it has 27 electrons. The electronic .

The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. Cobalt, as an electropositive element, willingly relinquishes its valence electrons during .
Iba pa The electron configuration of cobalt is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 7 4s 2. Cobalt, as an electropositive element, willingly relinquishes its valence electrons during .electronic configuration of co1 Answer. Meave60. Apr 16, 2015. Cobalt has an atomic number of 27, which means that its atoms have 27 protons in their nuclei. In a neutral cobalt atom, there is also 27 .
How do you write the electron configuration for cobalt, a transition metal? This example from the OpenChem course at UCI shows you the steps and the rules to follow, using .
The electron configuration of transition metals is special in the sense that they can be found in numerous oxidation states. Although the elements can display . Electron Configuration and Oxidation States of Cobalt. Electron configuration of Cobalt is [Ar] 3d7 4s2. Possible oxidation states are +2,3. Electron Configuration. The . Therefore, electron configuration of cobalt (Co 3+) ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6. Explanation of the transformation from Cobalt atom to Cobalt ion (Co 2+, Co 3+) Elements can have a positive or negative charge, except for noble gases. The element that accepts electrons while forming a bond is the electronegative element.
The electron configuration of "Co"^(3+) is ["Ar"] 4s 3d^5. "Co" is in Period 4 of the Periodic Table, and "Ar" is the preceding noble gas. Cobalt is also in Group 9, so it must have 9 valence electrons. The valence shell configuration is therefore 4s^2 3d^7, and the core notation is bb"Co": ["Ar"] 4s^2 3d^7 When a transition metal forms an ion, the s . Introduction to electron configurations. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of . Cobalt electron configuration diagram. The atomic number of cobalt is 27 which implies it has 27 electrons. The electronic configuration of Co diagrammatically is:. The first orbital is 1s which can hold 2 electrons (1s 2).; The second orbital is 2s which again has 2 electrons (2s 2).; The third orbital has the same energy shell and can withhold 6 .To check the answer, verify that the subscripts add up to the atomic number. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83).
Example of Determining Energy Levels (n) For example, if we want to determine the electron configuration for Cobalt (Co) at ground state, we would first look at the row number, which is 4 according to the periodic table below; meaning n = 4 for the s-orbital.In addition, since we know that the energy level for the d orbital is "n-1", therefore .
The s,p,d,f configuration for cobalt (Co) is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7, determined by the position of the element on the periodic table. Cobalt is an inner transition metal which means the electron configuration will end in a d block. Cobalt is in the 7th column of the d block and therefore has 7 d electrons d^7. The element cobalt . The electron configuration of the Cobalt can be represented as the [Ar] 3d7 4s2 in its most accurate and precise form. This electron configuration of the element represents the actual and the unique identity of the element. It further has a number of other significances in the domain of chemistry and quantum physics.
electronic configuration of co|Iba pa
PH0 · electronic configuration of zn
PH1 · electronic configuration of mn
PH2 · electronic configuration of cu
PH3 · electronic configuration of co molecule
PH4 · co3+ ground state electron configuration
PH5 · co 3+ electron configuration
PH6 · co 2+ electron configuration
PH7 · abbreviated electron configuration cobalt
PH8 · Iba pa